What is Atomic Mass?
You can find this storyboard in the following articles and resources:

Understanding Atomic Structures
Lesson Plans by Amy Roediger
Science teachers often rely on analogies and comparisons to help students picture what an atom “looks like”. The following activities aim to help students understand the atom, as it serves as the foundation for the rest of chemistry and plays an important role in many parts of physics.
'
Storyboard Description
What is atomic mass? How do we calculate atomic mass?
Storyboard Text
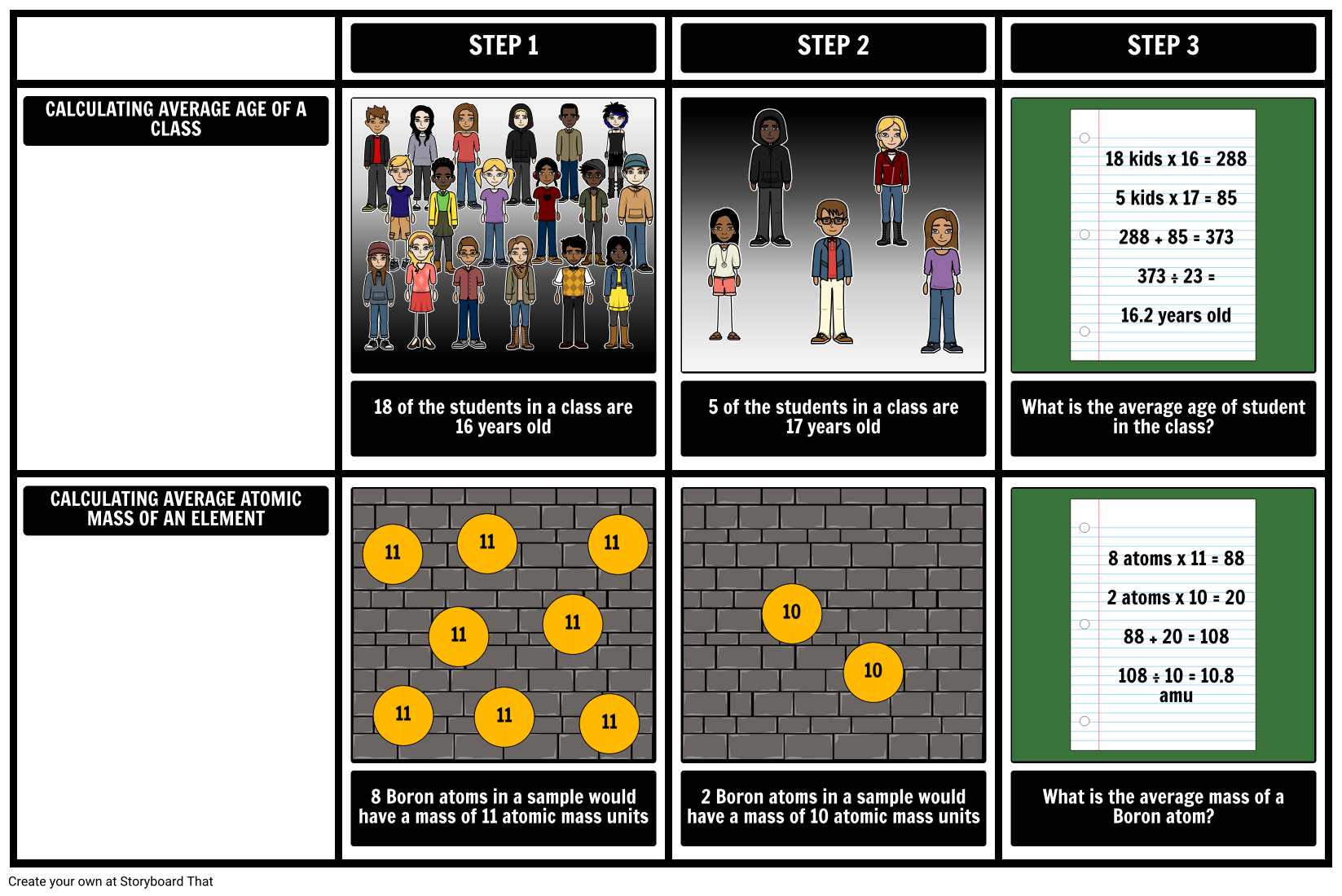
- CALCULATING AVERAGE AGE OF A CLASS
- STEP 1
- STEP 2
- STEP 3
- 18 kids x 16 = 288 5 kids x 17 = 85 288 + 85 = 373 373 ÷ 23 = 16.2 years old
- CALCULATING AVERAGE ATOMIC MASS OF AN ELEMENT
- 18 of the students in a class are 16 years old
- 11
- 11
- 11
- 5 of the students in a class are 17 years old
- What is the average age of student in the class?
- 8 atoms x 11 = 88 2 atoms x 10 = 20 88 + 20 = 108 108 ÷ 10 = 10.8 amu
- 8 Boron atoms in a sample would have a mass of 11 atomic mass units
- 11
- 11
- 11
- 11
- 11
- 2 Boron atoms in a sample would have a mass of 10 atomic mass units
- 10
- 10
- What is the average mass of a Boron atom?
Over 30 Million Storyboards Created








